| Title | TENTECH Secures Simultaneous Southeast Asian Certifications for 10TRIPLE, Expanding Global Market Entry | ||
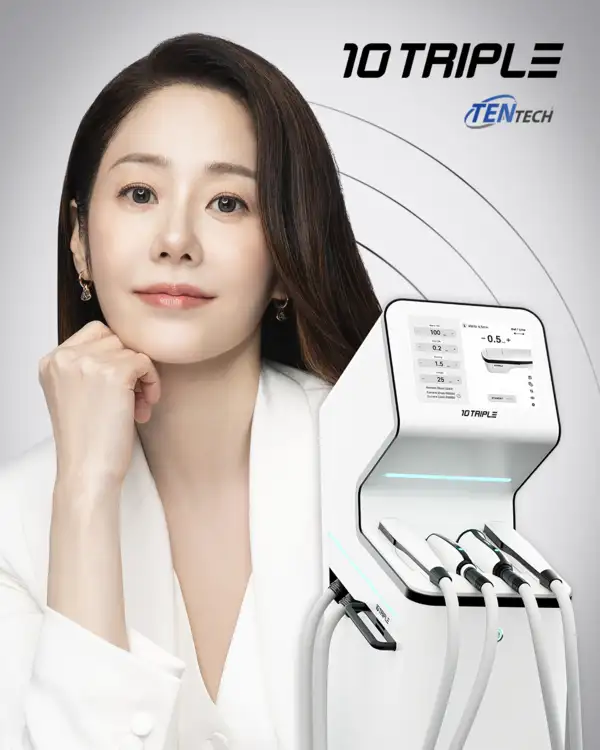
10THERMA Achieves European CE MDR Certification, Demonstrating Global Safety and Clinical Competitiveness TENTECH Co., Ltd. (CEO: Dong-ok Han), a specialized aesthetic medical device company, announced that it is accelerating its expansion into the global medical aesthetics market by securing medical device certifications in major Southeast Asian countries. TENTECH’s 3-line HIFU lifting device, 10TRIPLE, has recently obtained medical device certifications in Indonesia and Vietnam, establishing a solid foundation for entry into the Southeast Asian aesthetic medical market. Southeast Asia is experiencing rapid growth in demand for non-invasive lifting and skin tightening procedures, driven by its young population structure, expanding middle class, and strong interest in K-beauty. Following these certifications, TENTECH plans to strengthen collaboration with local partners and progressively roll out clinical and training programs for medical professionals, device demonstrations, and marketing campaigns centered around major cities in the region. Indonesia and Vietnam, in particular, are regarded as high-potential markets for aesthetic medicine. TENTECH aims to secure early reference clinics and hospitals, expand device supply, and establish a recurring revenue structure based on consumables, thereby laying the groundwork for stable local sales growth. Through this strategy, the company seeks not only short-term sales performance but also long-term market share expansion and a sustainable revenue model. Meanwhile, TENTECH’s monopolar radiofrequency (RF) medical device, 10THERMA, obtained the European CE MDR (Medical Device Regulation) certification in November last year, validating its global safety standards and clinical reliability. Compared to the former MDD, CE MDR imposes significantly stricter requirements for clinical data and safety evaluation and is considered one of the most rigorous certification processes for entry into the European market. With this certification, 10THERMA is expected to further accelerate its expansion not only in Europe but also in major global markets that recognize CE certification. A TENTECH official stated, “The Southeast Asian certifications for 10TRIPLE and the European CE MDR certification for 10THERMA are meaningful achievements that demonstrate TENTECH’s technological excellence meets global regulatory standards while also securing commercial competitiveness. Building on these global certifications, we plan to accelerate overseas sales growth in 2026 and strengthen our distribution, marketing, and clinical networks in key strategic markets to emerge as a leading global medical aesthetics company.” |
|||
| Hits | # 26 | Date | 2026.01.27 |
